

TNF-α: An In-Depth Analysis from Molecular Mechanisms to Clinical Applications
TNF-α: An In-Depth Analysis from Molecular Mechanisms to Clinical Applications
Tumor Necrosis Factor-alpha (TNF-α) is a pro-inflammatory cytokine secreted primarily by macrophages and monocytes, with molecular weights of 17.4 kDa or 26 kDa corresponding to the C-terminal extracellular domain of the full-length transmembrane protein. It can be produced by various cells, including adipocytes, activated monocytes, macrophages, B cells, T cells, and fibroblasts. TNF-α, mainly produced by macrophages, regulates cell proliferation, metabolic activation, inflammatory responses, and immune modulation.
I. Molecular Structure and Expression Regulation of TNF-α
TNF-α is a homotrimeric transmembrane protein composed of 233 amino acids. The mature TNF-α protein exists as a homotrimer, formed after the precursor protein (pro-TNF-α) is cleaved by TACE (TNF-α-converting enzyme) to release a soluble cytokine of 157 amino acids. Studies show that TNF-α expression is primarily regulated by the NF-κB and MAPK signaling pathways. Its expression is controlled at multiple levels: transcriptionally by factors like NF-κB and AP-1, and post-transcriptionally through mRNA stability mechanisms such as AU-rich elements (AREs).
From a structural perspective, TNF-α monomers adopt a β-folded "jelly-roll" conformation, with trimer assembly relying on hydrophobic interactions. This structure determines receptor-binding specificity and biological activity, with trimer stability directly influencing its half-life and function in vivo.
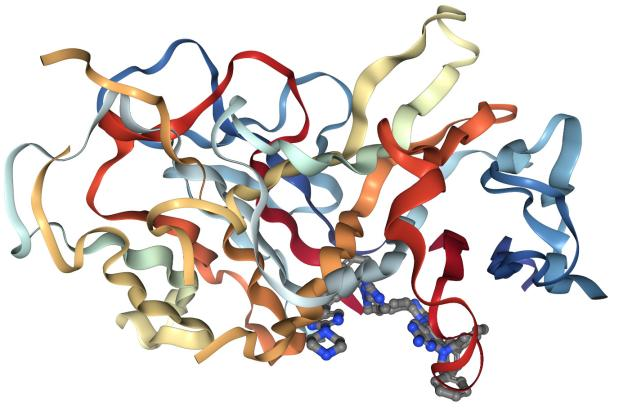
Caption: Schematic diagram of TNF-α's tertiary structure. macrophages and monocytes, with molecular weights of 17.4 kDa or 26 kDa corresponding to the C-terminal extracellular domain of the full-length transmembrane protein. It can be produced by various cells, including adipocytes, activated monocytes, macrophages, B cells, T cells, and fibroblasts. TNF-α, mainly produced by macrophages, regulates cell proliferation, metabolic activation, inflammatory responses, and immune modulation.
II. TNF-α Signaling Network
TNF-α exerts its biological functions through two transmembrane receptors: TNFR1 (p55) and TNFR2 (p75). TNFR1 is widely expressed across cell types, while TNFR2 is primarily found on immune and endothelial cells. Receptor activation triggers distinct downstream signaling pathways.
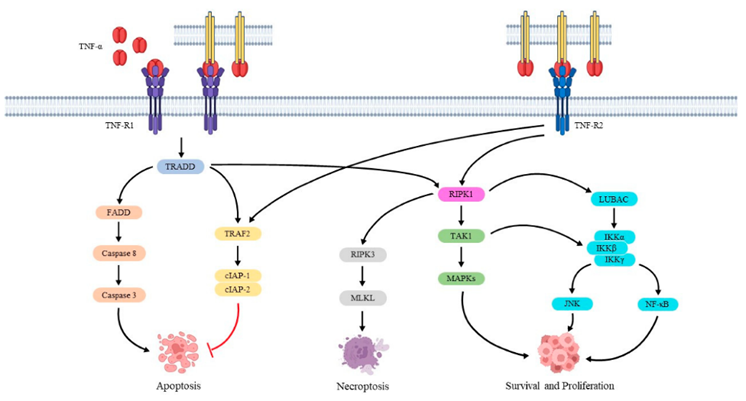
Caption: TNF-α signaling pathways mediated by TNFR1 and TNFR2.
Upon binding to TNFR1, TNF-α induces receptor trimerization and recruitment of the adaptor protein TRADD, activating two parallel pathways: one involving RIP1 and TRAF2 to activate NF-κB, and the other engaging FADD and caspase-8 to induce apoptosis. This dual-signaling mechanism allows TNF-α to exert opposing roles—promoting cell survival or programmed death—depending on the cellular context.
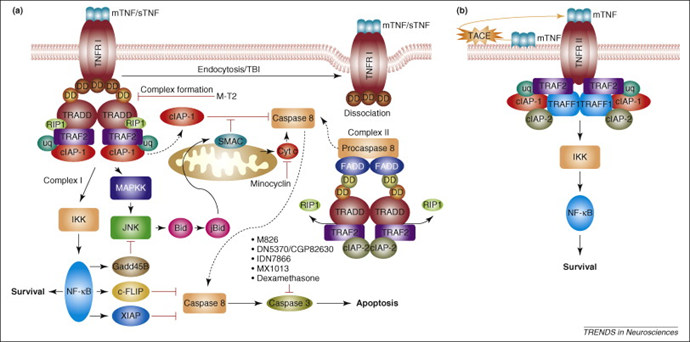
Caption: Schematic of TNF-α receptor signaling pathways.
III. Role of TNF-α in Disease Pathogenesis
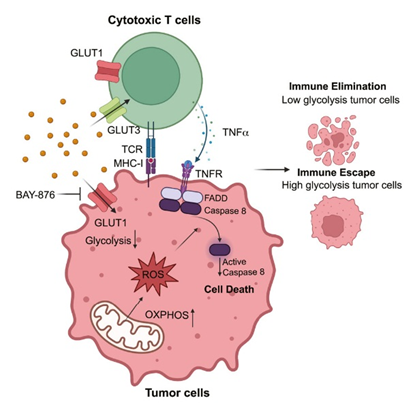
Dysregulated TNF-α expression is closely linked to various diseases. In rheumatoid arthritis, excessive TNF-α production by synovial cells induces matrix metalloproteinase expression, leading to cartilage destruction. In inflammatory bowel disease, TNF-α disrupts epithelial barrier function, exacerbating intestinal inflammation.
Recent studies reveal TNF-α’s role in metabolic diseases. In obese individuals, TNF-α secreted by adipose tissue macrophages interferes with tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1), inducing insulin resistance.
IV. Advances in TNF-α-Targeted Therapeutics
Given TNF-α’s central role in inflammatory diseases, several TNF-α inhibitors have been approved, including monoclonal antibodies (e.g., infliximab, adalimumab) and soluble receptor fusion proteins (e.g., etanercept). These agents differ subtly in mechanism: infliximab binds both soluble and transmembrane TNF-α, inducing apoptosis in cells expressing transmembrane TNF-α, while etanercept primarily neutralizes soluble TNF-α with lower affinity for transmembrane TNF-α. These differences impact clinical efficacy and safety profiles.
V. Detection Methods and ELK’s Innovations
Clinical laboratories predominantly use enzyme-linked immunosorbent assays (ELISA) for TNF-α quantification, endorsed as the standard method in the 2021 Clinical Testing Guidelines. Elevated TNF-α levels are observed in sepsis, malignancies, heart failure, and chronic inflammatory diseases.
ELISA relies on antigen-antibody reactions, with enzyme-conjugated secondary antibodies generating detectable signals for quantitative analysis. ELK’s TNF-α ELISA kit incorporates key innovations:
High-Affinity Monoclonal Antibody Pairs: Minimize cross-reactivity with other cytokines (e.g., TNF-β).
Signal Amplification System: Lowers the detection limit to 5.7 pg/mL, ideal for low-abundance samples.
Versatile Sample Compatibility: Supports diverse sample types (e.g., body fluids, tissue homogenates) with detailed pre-processing guidelines.
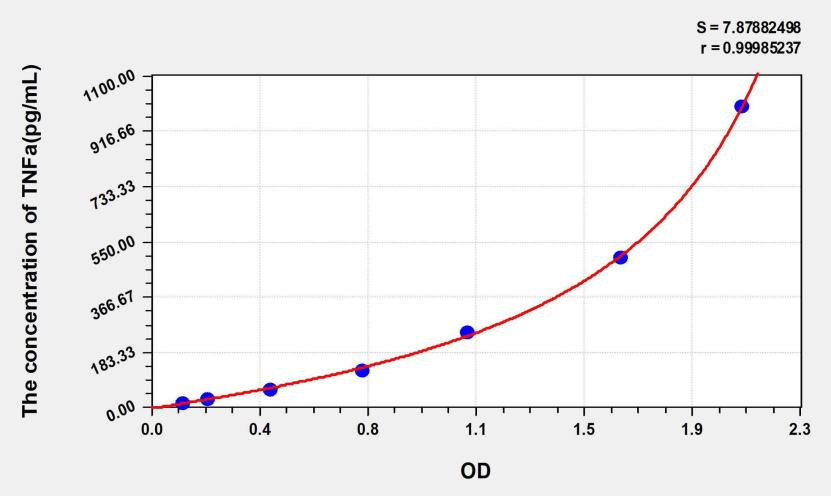
Caption: Standard curve for TNF-α quantification, demonstrating high sensitivity (r ≈ 0.9999).


