

Mechanisms of ferroptosis in the treatment of liver fibrosis
Mechanisms of ferroptosis in the treatment of liver fibrosis
A new type of programmed cell death called ferroptosis was first proposed by Brent R. Stockwell's group at Columbia University in 2012. Since then, the research on ferroptosis has been increasing year by year. This issue of ELK Biotechnology will lead us to explore the mechanism of ferroptosis regulation and liver fibrosis.
What is ferroptosis
Unlike cells apoptosis and autophagy, death is a kind of dependence on iron iron process. The essence of ferroptosis is the depletion of glutathione (GSH) and the reduction of glutathione peroxidase 4(GPX4) activity. When glutathione peroxides cannot be reduced by GPX4, lipid peroxides will not be metabolized, thereby destroying the integrity of the cell membrane and leading to cell death. Ferroptosis is characterized by its unique morphology, biochemistry and genetics.
● Morphological characteristics
Show the mitochondria shrinkage, double membrane density increased, mitochondrial cristae reduced or disappeared, the mitochondrial outer membrane rupture; The nuclei were of normal size without chromatin aggregation.
Low and biochemical characteristics
Iron accumulation, lipid peroxidation, activation of the mitogen-activated protease (MAPKs) System, inhibition of cystine glutamate antitransporter (System Xc-, a heterodimer composed of SLC3A2 of SLC7A11) decreased cystine uptake, glutathione (GSH) depletion, increased oxidation of NAPDH, Release arachidonic acid mediators such as 11-HETE and 15-HETE.
● Immune characteristics
Release damage-associated molecular patterns (DAMPs) and promote inflammatory responses.
Iron metabolism and ferroptosis
Iron is an indispensable trace element in the human body and plays an important role in normal physiological functions. Iron is involved in a variety of physiological processes within the cell, such as oxygen transport, mitochondrial respiration, DNA replication, and cell signaling. However, iron has limited bioavailability because it exists mainly as Fe3+ ions, which are insoluble in aqueous solutions. Excess free Fe2+ ions may trigger oxidative stress and lipid peroxidation. Iron content inside and outside the cell is controlled by regulatory systems through transferrin import and ferroportin export. Increasing the iron content of the variable iron pool increases susceptibility to oxidative damage and ferroptosis. Through inhibition or induction of iron death regulation and control, found that iron death may be liver fibrosis in liver disease diagnosis, prevention and treatment of potential targets.
Ferroptosis and liver fibrosis
Fibrotic diseases develop from a complex pathological process in which cells undergo sustained chronic damage. Ferroptosis-related mechanisms play an important regulatory role in the progression of fibrotic diseases. Hepatic fibrosis is the main pathological characteristics of excessive activation of HSC and the ECM deposition in the liver, portal area abundant fibrous connective tissue hyperplasia, can damage the liver normal structure and physiological function. Therefore, inhibiting the activation and proliferation of HSC and inducing the death of HSC are effective methods for the treatment of liver fibrosis. Studies have shown that store has a lot of iron ion in HSC, iron death can through regulating HSC iron ion content and degree of lipid peroxidation influence the development of liver fibrosis process, thus targeted HSC iron death may become the new strategy for treatment of liver fibrosis [1].
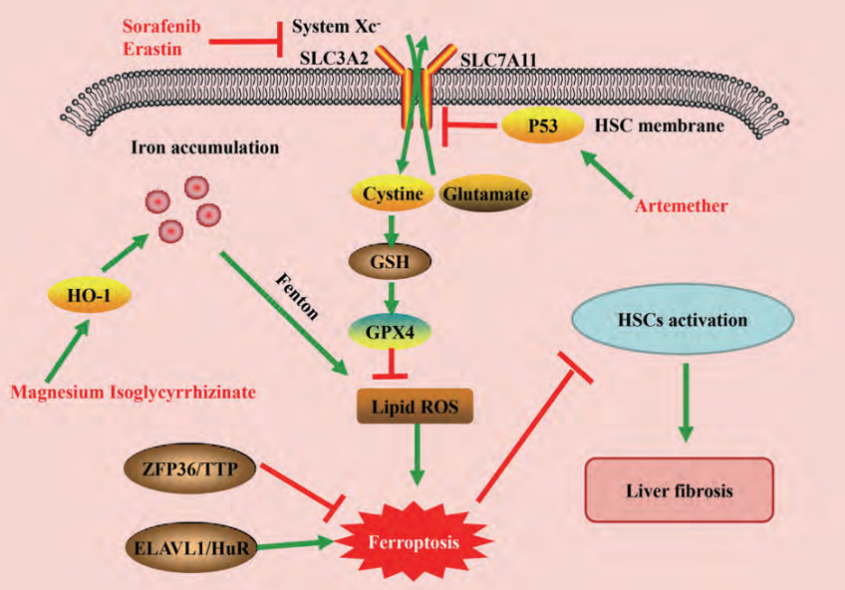
Figure 1: Relevant regulatory pathways of ferroptosis in liver fibrosis
Iron death role in liver fibrosis and molecular mechanism
01 RNA-binding protein ZFP36 / TTP and ELAVL1 / HuR control iron death
Sui et al. [2] found that in the rat liver fibrosis model, magnesium isoglycyrrhizinate can induce the up-regulation of HO-1, promote the accumulation of iron and lipid peroxides, and thus promote the occurrence of ferroptosis of HSCS. Treatment with ferroptosis inhibitor ferrostatin-1 or silencing HO-1 abolished the anti-fibrotic effect of magnesium isoglycyrrhizinate. ZFP36 ring finger protein 36(ZFP36 /TTP) and ELAV like RNA binding protein 1(ELAV like RNA binding protein 1, ELAV like RNA binding protein 1, ZFP36/TTP) ELAVL1/HuR) played an important role in regulating HSC ferroptosis. Down-regulation of ZFP36 and up-regulation of ELAVL1 promoted sorafenib/Erastin-mediated HSC ferroptosis and alleviated liver fibrosis in mice. Further mechanism study found ZFP36 / ELAVL1 may combine with downstream target genes, affecting its mRNA stability, thus regulating HSC ChuTie proteins in autophagy, release of iron ions, which in turn by fenton reaction produces a large number of ROS, promote the HSC iron death [3, 4].
02 The BRD7-P53-SLC25A28 axis and Trf regulate ferroptosis
Zhang et al. [5] found that BRD7 knockout mediated by CRISPR/cas9 can reduce HSC ferroptosis, while BRD7 overexpression mediated by specific BRD7 plasmid can promote HSC ferroptosis. Furthermore, we found that BRD7 mediated ferroptosis by directly binding to P53 N-terminal transactivation domain to promote P53 mitochondrial translocation, thereby interacting with downstream SLC25A28 to form a complex. The researchers found that mice liver cells specificity knockout agency performance for the liver of transferrin in combination with iron accumulation, increase high iron diet mediated liver fibrosis, and at the same time specific knockout mice liver cells agency and solute carrier family 39 members of 14 (solute carrier family 39 member 14, SLC39A14) can significantly reduce liver iron accumulation and ameliorate liver fibrosis mediated by high-iron diet or carbon tetrachloride injection, these data suggest that liver transferrin plays a protective role in maintaining liver function, providing a potential therapeutic target for the prevention of iron overload-induced liver fibrosis [6].
03 P62 - Keap1 - Nrf2 signaling pathways regulating iron death
Current studies suggest that the final execution mechanism of iron death is caused by excessive accumulation of lipid peroxides plasma membrane damage, caused the occurrence of iron cell death. Sun et al. [7] reported that silencing P62 expression could increase the ferroptosis of erastin/ sorafenafeni-induced hepatocellular carcinoma (HCC) cells. In addition, in HCC cells transplantation tumor model in mice, and found in the experiments, the nuclear factor E2 - related factor 2 (nuclear factor erythroid 2 - related factor 2. Nrf2) on low also show enhanced erastin/sorafenib resistance of HCC. These results suggest that the p62-Kelch-like ECH-associating protein 1(Keap1)-Nrf2 signaling pathway may play an important role in ferroptosis. Nrf2 may be involved in iron, therefore, death and future important targets for the treatment of a variety of liver diseases.
Ferroptosis inducers, inhibitors, and liver fibrosis
01 targeting system Xc-induced ferroptosis
Current research and development have found that ferroptosis inducers mainly induce ferroptosis by targeting system Xc-, GSH, GPX4, iron ions and ROS. Among them, erastin is the first identified specific ferroptosis inducer, which can target the inhibition of system Xc- activity and affect GSH synthesis. It promotes ferroptosis in a variety of cells [8].
02 targets iron ions and ROS to induce ferroptosis
Studies have shown that a variety of traditional Chinese medicine, such as artemisinin and artemipiplumine, can induce ferroptosis by increasing ROS content and thereby affecting iron metabolism, which plays an anti-liver cancer effect with low toxicity and are very promising drugs [9]. FINO2 is a kind of plakinic acid D derivative with 1, 2-dioxopentane structure, which can induce cell ferrodeath through Fenton reaction, indirect inactivation of GPX4 and direct oxidation of PUFA, and its effect is stronger in tumor cells with high iron level [10].
03 Targeting GSH to induce ferroptosis
Butylcystimide promotes lipid peroxidation by inhibiting the synthesis of GSH rate-limiting enzyme gluta‐mate-cysteine ligase (GCL) and reducing GSH level and GPX4 activity, thereby inducing ferroptosis in a variety of cancer cells. Cisplatin can directly bind to GSH to form Pt(Pt)-GSH complex, which leads to the inactivation of GSH and GXP4, thereby promoting ferroptosis. Researchers further used cisplatin and erastin in combination on human lung cancer cells and human colon cancer cells and found that the combined drugs showed significant synergistic anti-tumor effect [11].
04 Targets lipid peroxidation to inhibit ferroptosis
Brown rice extract can improve lipid peroxidation and cytotoxicity caused by GPX4 inactivation by inhibiting ferroptosis [12]. Baicalin can also inhibit ferroptosis by inhibiting lipid peroxidation and reducing free iron accumulation, and its inhibitory effect is significantly better than some typical ferroptosis inhibitors [13].
05 targets ACSL4 to inhibit ferroptosis
Thiazolidinediones such as rosiglitazone, pioglitazone and troglitazone can specifically inhibit the expression of ACSL4, thereby protecting cells from RSL3-induced ferroptosis and lipid peroxidation. Among them, troglitazone has the strongest inhibitory effect on ferroptosis among thiazolidinediones, although it has a low inhibitory effect on ACSL4, it may have inherent antioxidant activity due to its 6-chromogenol structure [14]. Some researchers have also combined nanotechnology with ferroptosis research, using nanomaterials to inhibit or induce ferroptosis. Therefore, it is of great significance to explore the mechanism of ferroptosis inducers and inhibitors and to develop new drugs targeting ferroptosis for the treatment of liver fibrosis.
Ferroptosis related products are recommended
As a supplier of protein antibodies in the field of life science and medicine, ELK biotechnology can provide high-quality reagents for ferroptosis related research. Here, some ferroptosis products are listed for your reference. For details, see the table below:
|
Cat.No |
Name |
Application |
Species |
|
ELK1206 |
Rat TRF(Transferrin) ELISA Kit |
ELISA |
Rat |
|
ELK1300 |
Human TRF(Transferrin) ELISA Kit |
ELISA |
Human |
|
ELK1330 |
Mouse TRF(Transferrin) ELISA Kit |
ELISA |
Mouse |
|
ELK1356 |
Dog TRF(Transferrin) ELISA Kit |
ELISA |
Dog |
|
ELK8013 |
Pig TRF(Transferrin) ELISA Kit |
ELISA |
Pig |
|
EM1231 |
Transferrin Mouse mAb |
WB; IHC; ELISA |
Human |
|
EA188 |
Transferrin Rabbit pAb |
WB; IHC |
Human |
|
ES3967 |
Transferrin rabbit pAb |
WB; IHC; IF; ELISA |
Human;Mouse;Rat |
|
ELK1214 |
Human FE(Ferritin) ELISA Kit |
ELISA |
Human |
|
ELK3783 |
Rat FE(Ferritin) ELISA Kit |
ELISA |
Rat |
|
ELK5240 |
Mouse FE(Ferritin) ELISA Kit |
ELISA |
Mouse |
|
ELK6036 |
Pig FE(Ferritin) ELISA Kit |
ELISA |
Pig |
|
ELK9108 |
Dog FE(Ferritin) ELISA Kit |
ELISA |
Dog |
|
ES2337 |
Fer rabbit pAb |
WB; IHC; IF; ELISA |
Human;Mouse;Rat |
|
ES5400 |
Ferritin heavy chain rabbit pAb |
WB; ELISA |
Human;Mouse;Rat |
|
ELK0719 |
Rat GPX4(Glutathione Peroxidase 4) ELISA Kit |
ELISA |
Rat |
|
ELK4775 |
Human GPX4(Glutathione Peroxidase 4) ELISA Kit |
ELISA |
Human |
|
ELK8786 |
Mouse GPX4(Glutathione Peroxidase 4) ELISA Kit |
ELISA |
Mouse |
|
ELK2939 |
Human GRP78(Glucose Regulated Protein 78) ELISA Kit |
ELISA |
Human |
|
ELK6448 |
Mouse GRP78(Glucose Regulated Protein 78) ELISA Kit |
ELISA |
Mouse |
|
ELK6834 |
Rat GRP78(Glucose Regulated Protein 78) ELISA Kit |
ELISA |
Rat |
|
ELK1665 |
Mouse COL4(Collagen Type Iv) ELISA Kit |
ELISA |
Mouse |
|
ELK1712 |
Rat COL4(Collagen Type Iv) ELISA Kit |
ELISA |
Rat |
|
ELK2331 |
Human COL4(Collagen Type Iv) ELISA Kit |
ELISA |
Human |
【References】
[1] Anderson ER, Shah YM. Iron homeostasis in the liver [J]. Compr Physiol, 2013, 3: 315-330.
[2] Sui M, Jiang X, Chen J, et al. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway [J]. Biomed Pharmaco‐ ther, 2018, 106: 125-133.
[3] Zhang Z, Yao Z, Wang L, et al. Activation of ferritinophagy is required for the
RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells [J]. Autophagy, 2018, 14: 2083-2103.
[4] Zhang Z, Guo M, Li Y, et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells [J]. Autophagy, 2020, 16: 1482- 1505.
[5] Zhang Z, Guo M, Shen M, et al. The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells [J]. Redox Biol, 2020, 36: 101619.
[6] Yu Y, Jiang L, Wang H, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis [J]. Blood, 2020, 136: 726-739.
[7] Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells [J]. Hepatology, 2016, 63: 173-184.
[8] Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death [J]. Cell, 2012, 149: 1060-1072.
[9] Ooko E, Saeed ME, Kadioglu O, et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells [J]. Phytomedicine, 2015, 22: 1045-1054.
[10] Abrams RP, Carroll WL, Woerpel KA. Five-membered ring peroxide selectively initiatesferroptosis in cancer cells [J]. ACS Chem Biol, 2016, 11: 1305-1312.
[11] Guo J, Xu B, Han Q, et al. Ferroptosis: a novel anti tumor action for cisplatin [J]. Cancer Res Treat, 2018, 50: 445-460.
[12] Xu WH, Li CH, Jiang TL. Ferroptosis pathway andits interven‐ tion regulated by Chinese materia medica [J]. China J Chin Mater Med (中国中药杂志), 2018, 43: 4019-4026.
[13] Xie Y, Song X, Sun X, et al. Identification of baicalein as a ferroptosis inhibitor by natural product library screening [J]. Biochen Biophys Res Commun, 2016, 473: 775-780.
[14] Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition [J]. Nat Chem Biol, 2017, 13: 91-98.
[15] Chen Hao, Li Xiaofeng, Wang Hua. Research progress on the mechanism of iron death in regulating liver fibrosis [J]. Acta Pharmacologica Sinica, 2019,56(11):2916-2922.DOI:10.16438/J.0513-4870.2021-0959.


