Transforming growth factor beta(chapter 2)

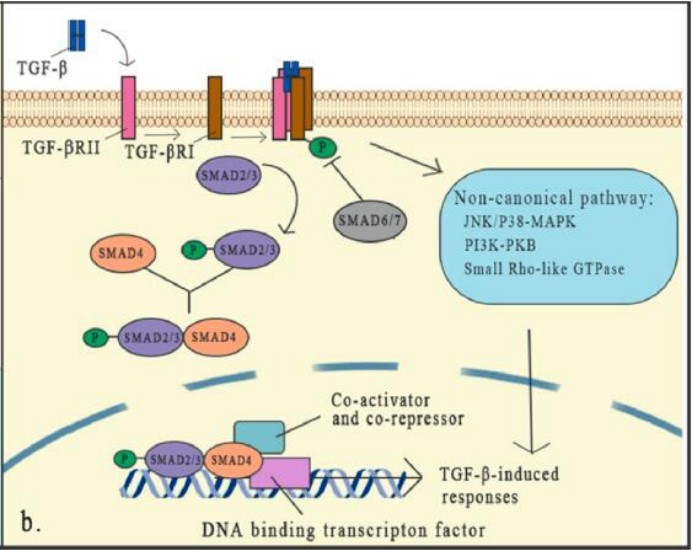
TGF-β signal channel
TGF-β via TGF-βi-type receptor (TGF-βRi or ALK5) and TGF-βII receptor (TGF-βRii) triggering signal conduction. Both of these receptors are transgeotic deroyedine/soverenate kinase receptors. Essence
After combining TGF-β, TGF-βRii recruits the intracellular domain of TGF-βri as a high-affinity TGF-β receptor to form a heteroothye, and then activate the SMAD protein of the downstream signal.
SMAD's classic pathway is the core part of the TGFβ signal conduction. There are eight SMAD proteins in mammals in three categories, including receptor-related SMADS (R-SMADS), collaborative SMADS (CO-SMADS), and inhibitory SMAD (I-SMADS).
Activation of TGF-β and receptor activation can cause R-SMADS to activate. Once activated, SMAD2/3 is separated from the receptor and forms an heterogeneous trimer structure with SMAD4, and then transferred to the nucleus, where they are transcribed with DNA transcription Factors and auxiliary factors are combined to activate or inhibit hundreds of target genes.
The expression of I-SMADS is induced by the TGF-β signal as the feedback reaction. Smads usually activate or inhibit SMADS through phosphorylation of different kinases and generalization of different plastic enzymes to control the stability and activity of SMADS. SMAD6 and SMAD7 reduces the phosphorylation and activation of R-SMAD by combining with R-SMADS competition and TGF-β receptor complexes.
In addition to the typical approach of TGF-β signal transition, it has been thoroughly studied and proved to have an important role, the non-classic (non-SMAD) signal pathway still exists, but people know less about it. Although the core mechanism of the TGF-β signal transition has been well studied, the interaction of this signal and specific factor and other signal pathways forms a dense network in the TGF-β-level joint regulation, which indicates that TGF The role of -β in the micro -ring is complicated. Any cell signal that affects the TGF-β signal conduction element will change its effectiveness.
The role of TGF-β in tumor microenvironment
TGF-β plays a dual role in the TME, which is determined by the different stages of tumor development and in the context of genetic alterations. In early-stage tumors, the TGF-β pathway induces apoptosis and inhibits cell proliferation, including cancer cells.
Paradoxically, at advanced stages, it has pro-tumor effects by regulating genomic instability, EMT, neovascularization, immune evasion, and metastasis. When pro-tumor function overwhelms the anti-tumor effects of TGF-beta signaling in cells, it acts in a consistent manner as a tumor-promoting agent.
4.1 TGF-β induces cell cycle arrest and apoptosis
Studies have shown that TGF-β exposure promotes cell cycle arrest through different mechanisms in a variety of cultured eukaryotic cells, including epithelial cells, endothelial cells, hematopoietic cells, and skin keratinocytes.
TGF-β-mediated growth arrest inhibition usually occurs in the G1 phase of the cell cycle, when cells are sensitized to TGF-β. One of the important mechanisms is that TGF-β inhibits the transcription of MYC, which is required for the progression from G1 to S phase.
4.2 Changes in TGF-β signaling in tumor cells
Numerous genetic and epigenetic alterations are found in many cancers that lead to an autonomous switch of TGF-β signaling from tumor suppression to promotion.
Numerous studies have shown that SMAD2, SMAD3, SMAD4, TGF-βRI, and TGF-βRII are mutated or deleted in many tumor types. SMAD4 is the most common alteration and plays a dominant role in carcinogenesis, and its mutations lead to loss of tumor suppressive effects of multiple TGF-β superfamily members. SMAD2 gene mutations have also been reported in hepatocellular carcinoma (HCC), colorectal cancer and lung cancer.
4.3 The regulatory effect of TGF-β on tumor EMT
It is generally accepted that TGF-β-induced EMT is an important step in tumor invasion and metastasis. E-cadherin is a key protein that maintains epithelial cell adhesion to adherens junctions. TGF-β induces the expression of transcription factors SNAIL, ZEB and TWIST, and inhibits the expression of E-cadherin, thereby regulating cell adhesion, polarity Sex and reorganization of the cytoskeleton.
Furthermore, TGF-β enhances the activity of metalloproteinases (MMPs), allowing remodeling of the cytoskeleton.
4.4 The role of TGF-β in angiogenesis
TGF-β has been shown to stimulate angiogenesis and act as a pro-angiogenic factor. TGF-β induces the expression of vascular endothelial growth factor (VEGF), an important angiogenic factor in vitro.
In glioblastoma, HCC, and CRC models, treatment with inhibitors of the TGF-β pathway reduced microvessel density and inhibited tumor vascularization. In a diffuse-type gastric cancer model, disruption of TGF-β signaling appears to accelerate tumor growth by increasing tumor angiogenesis. Therefore, the promoting role of TGF-β in angiogenesis depends on the tumor type and environment.
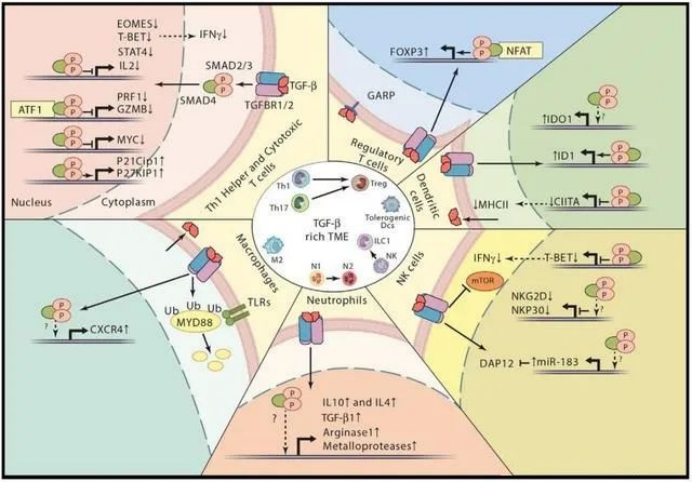
The role of TGF-β in the immune system
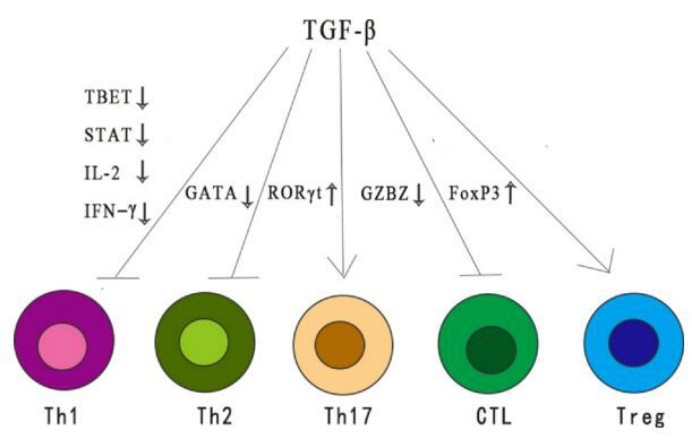
TGF-β, as an immunosuppress cytokine, has a wide range of inhibitory effects on immune response through different mechanisms. TGF-β reduces the differentiation and functions of TH1, TH2 cells, and cytotoxic T lymphocytes (CTL). All these provide important anti-tumor reactions.
TGF-β also enhances immune tolerance and tumor escape by regulating the number and function of regulatory T (TREG) cells. In addition, TGF-β regulates the fate of immune cells by inhibiting or stimulating cell proliferation, which affects the development of thymus and peripheral T cells.
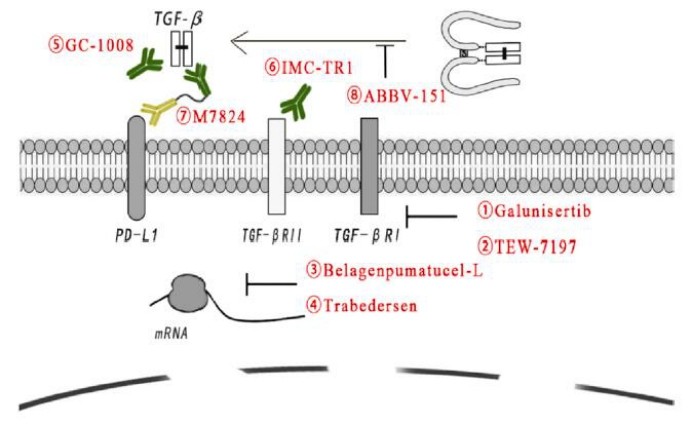
Tumor-targeted therapy of TGF-β
Although many factors add to the complexity of TGF-β signaling, drugs targeting TGF-β signaling have shown potent activity in tumor therapy. In addition, the clinical success of ICIs has accelerated interest in harnessing TGF-β signaling as an enhanced anticancer therapy.
These specific or nonspecific drugs include small molecules targeting the TGF-beta receptor, antisense oligonucleotides, vaccines, neutralizing antibodies, and receptor IgG-Fc fusion proteins.