

Alzheimer's Disease (AD) related indicators - Tau protein
Background: Tau protein is a low molecular weight microtubule associated protein (MAP) discovered in the mid-1970s through research on factors required for microtubule formation. It is primarily distributed in the central nervous system, mostly found in the axons of neurons, with a smaller presence in oligodendrocytes. Tau protein has a molecular weight of 45-50 kDa and contains 352-441 amino acids. Its classic biological function is to promote microtubule assembly and maintain microtubule stability. When Tau protein undergoes hyperphosphorylation, abnormal glycosylation, aberrant glycation, and ubiquitination, it loses its stabilizing effect on microtubules, leading to neurofibrillary degeneration and functional loss. Tau protein is a major component of neurofibrillary tangles and a marker for a series of neurodegenerative diseases, including Alzheimer's Disease (AD).
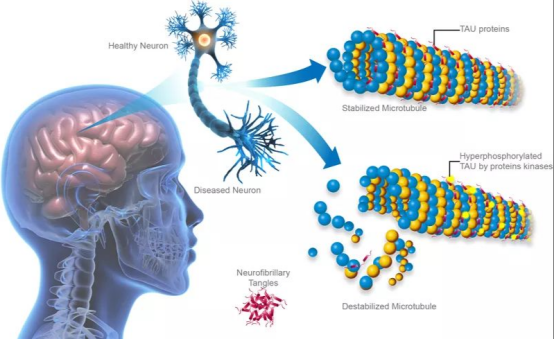
This image is sourced from the journal 'Science Advances'.
Introduction:
Structural Features of Tau ProteinThe Tau protein is composed of four main structural domains: the N-terminal projection region, the proline-rich region, the microtubule-binding domain (MBD), and the C-terminal region. The N-terminal projection region interacts with other cytoskeletal components and the cell membrane, playing an important role in maintaining axonal stability; the proline-rich region contains multiple phosphorylation sites involved in the functional regulation of neurotransmission and intracellular signaling; the MBD determines the Tau protein's ability to bind to microtubules; the C-terminal region complements the function of the MBD, together maintaining the stability and function of microtubules. Through alternative splicing, the Tau protein can generate six major isoforms, with differences arising from the presence of N-terminal insertion sequences (0N, 1N, 2N) and MBD repeat sequences (3R or 4R). These variations result in functional differences among the various Tau isoforms, particularly in their ability to bind to microtubules.
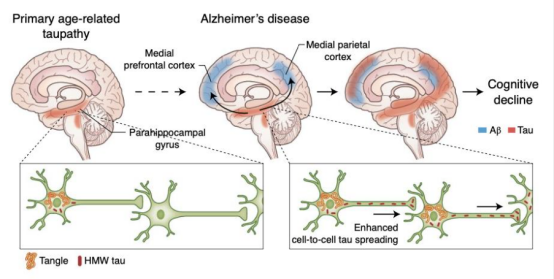
This image is sourced from the article on Alzheimer’s disease in The Lancet/Neurology.
The expression and classification of Tau protein
Tau is encoded by the MAPT gene on chromosome 17, with a gene length of over 100 kb, containing 16 exons. Exon 1 is part of the promoter and is transcribed but not translated. Exons 1, 4, 5, 7, 9, 11, 12, and 13 are constitutive exons. Exons 2, 3, and 10 undergo alternative splicing, where exon 2 can occur independently, but exon 3 cannot occur without exon 2.In the central nervous system, selective splicing of exons 2, 3, and 10 produces six isoforms of Tau: 0N3R, 1N3R, 2N3R, 0N4R, 1N4R, and 2N4R Tau. Based on solubility and phosphorylation levels, Tau can be categorized into: soluble non-abnormal phosphorylated Tau (C-Tau), soluble abnormal phosphorylated Tau (ADp-Tau), and insoluble Tau aggregated into paired helical filaments (PHF-Tau). C-Tau's biological activity is similar to that of normal Tau protein; ADP-Tau has no biological activity but has not aggregated into PHF; PHF-Tau is the abnormally hyperphosphorylated Tau protein extracted from neurofibrillary tangles.
Alzheimer's disease and Tau protein
Alzheimer's disease (AD) is the most common and complex neurodegenerative disorder among the elderly, with one of its significant pathological features being the abnormal phosphorylation of Tau protein. The Tau protein dissociates from microtubules, forming disordered aggregations that ultimately result in neurofibrillary tangles, which impair the structure and function of neurons, leading to memory loss and cognitive impairment. The accumulation of β-amyloid (Aβ) is another important pathological characteristic of AD. Research indicates that the pathological changes in Tau are closely related to the accumulation of Aβ. In the early stages of AD, Aβ accumulates between neurons and forms plaques; these Aβ plaques indirectly promote the phosphorylation and aggregation of Tau through the activation of inflammatory responses and the induction of oxidative stress. The aggregation of Tau may, in turn, exacerbate the toxicity of Aβ, creating a vicious cycle that drives the pathological progression of AD.
Biological functions of P-tau protein
Neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein (p-tau) are one of the hallmark pathological features of Alzheimer's disease (AD). p-tau181 in cerebrospinal fluid has been regarded as a biomarker for NFTs and has been used to support the clinical diagnosis of AD and the prediction of mild cognitive impairment. However, many phosphorylated sites of p-tau, including p-tau181, p-tau217, and p-tau231, have shown potential to serve as diagnostic markers for AD. Some of these types of p-tau do not show consistent expression in cerebrospinal fluid compared to plasma and brain, while the enrichment of p-tau in cerebrospinal fluid and plasma is believed to be driven by amyloid proteins, indicating more about the pathology of AD.


